What happened?
Enztec’s experience came to the fore in the creation of the X-WING◊ Graft Preparation System, driving it forward with speed and attention to detail.
During the design phase, Enztec collaborated closely with Smith+Nephew to refine key product requirements: the new product’s capabilities, its design constraints and how it would differ from the currently available alternatives. The product began to take shape after all of the relevant factors were considered.
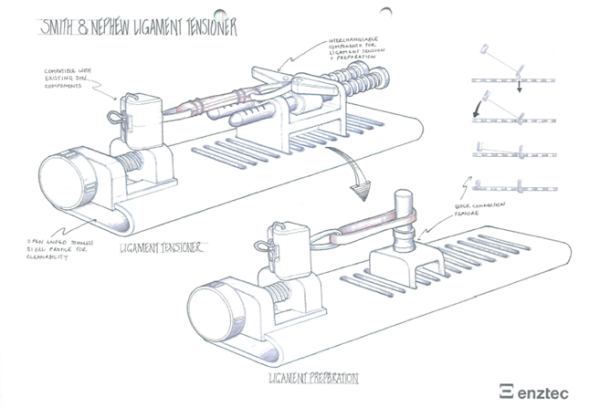
In terms of verification and validation, extensive testing was carried out by Enztec to ensure the mechanisms and material couplings selected for this high-load tensioning application were suitable. Enztec also managed the clinical evaluation in New Zealand, allowing the designers to get feedback firsthand, which sped up the evaluation cycle.
Enztec created and openly shared all technical documentation, which allowed Smith+Nephew to adopt the new product easily and seamlessly into their system. Enztec and Smith+Nephew then co-developed a surgical technique to support the procedure.
As the manufacturer of record for X-WING◊, Enztec is responsible for delivering high-quality products that are designed to observe U.S. FDA, Australian TGA and EU MDR regulations.
Since its official release, Enztec has worked collaboratively with the Smith+Nephew team to match manufacturing capacity with demand. Forecasting from Smith+Nephew allows Enztec to be responsive in manufacturing and has helped to greatly reduce lead times.
X-WING◊ was initially deployed in Australia and New Zealand, where it helped Smith+Nephew differentiate itself from competitors and gain market share. The product soon became globally recognized and has since been released in a number of key international markets.